University of Maryland
- Pfizer is piloting delivery options for its COVID-19 vaccine in Rhode Island, Texas, New Mexico, and Tennessee, Reuters reported Tuesday.
- The vaccine has to be shipped and stored at minus 94 degrees Fahrenheit, which poses major distribution challenges. Moderna’s vaccine, by comparison, can be stored in a fridge.
- The four states in the trial won’t be given priority access to the vaccine.
- States have been preparing for the vaccine’s approval by buying hyper-cold freezers to store in it.
- Visit Business Insider’s homepage for more stories.
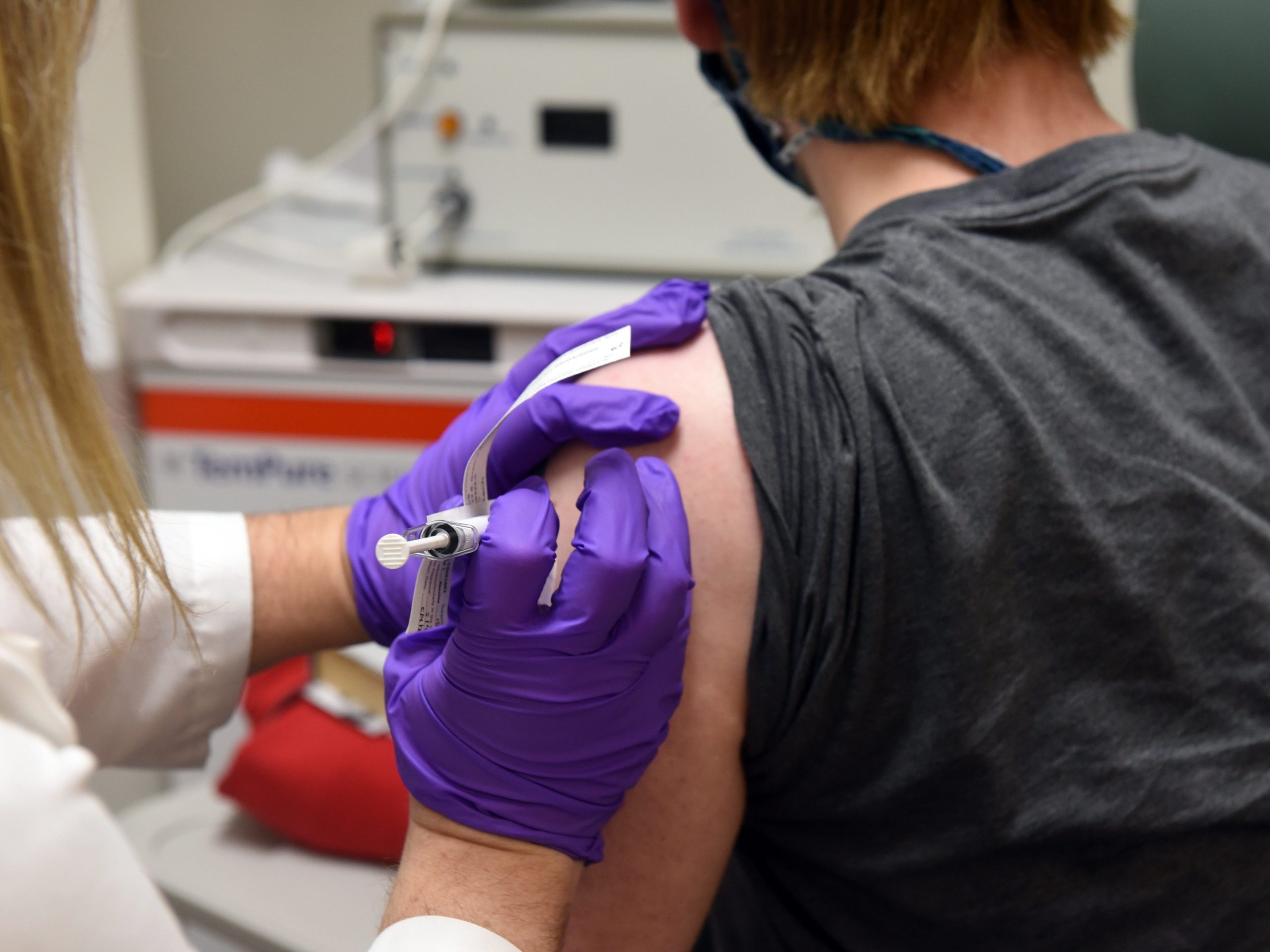
Pfizer has launched a pilot in Rhode Island, Texas, New Mexico, and Tennessee to test distribution of its COVID-19 vaccine, which has to be kept at temperatures well below freezing.
Pfizer’s vaccine candidate has to be shipped and stored at minus 94 degrees Fahrenheit (minus 70 degrees Celsius). Its distribution therefore relies on a “cold chain,” a multi-part pipeline keeps the shots chilled, from manufacturer to injection.
Most vaccines — including Moderna’s — are stored at around 35 to 46 degrees Fahrenheit.
States have been rushing to buy hyper-cold freezers for the vaccine, which could be available to all Americans by April 2021.
UBS analysts have predicted that roughly between 5% and 10% of the Pfizer vaccine may be rendered ineffective “due to inadequate storage conditions.”
Pfizer chose the four states for its pilot based on differences in their size, population diversity, and immunization infrastructure, Reuters reported. The states also have a mix of rural and urban populations.
"We are hopeful that results from this vaccine delivery pilot will serve as the model for other US states and international governments, as they prepare to implement effective COVID-19 vaccine programs," Pfizer said in a statement on Monday, per Reuters.
The four states will not get vaccine doses earlier than any other states because of the pilot, Pfizer said.
Last week, Pfizer announced that its experimental coronavirus vaccine succeeded in the final stage of clinical trials, marking a milestone in the fight against the pandemic. The drugmaker said the shot was found to be more than 90% effective in preventing COVID-19.
Around a third of US states are already purchasing ultra-cold storage equipment. Manufacturers are anticipating months-long back-orders and delays for the products — which can range in price from $5,000 to $15,000 — due to unprecedented demand, Reuters reported.
On Monday, Moderna announced that its vaccine candidate was 94.5% effective in late-stage trials. Unlike Pfizer's vaccine, Moderna's vaccine is stable for up to a month at standard refrigeration temperatures.